GenoType CMdirect VER 1.0
Your test system for the detection of M. tuberculosis complex and differentiation of more than 20 clinically relevant NTM directly from patient specimens
The members of M. tuberculosis complex are considered to cause tuberculosis. In contrast, the group of nontuberculous mycobacteria (NTM) comprises numerous species that are mostly less pathogenic. However, there are some NTM that can cause certain diseases. Thus, the differentiation of tuberculosis (TB) pathogens and NTM plays an important role for diagnostics, treatment and infection prevention.
NTM are found in different environmental compartments such as soil and drinking water or in food, like cheese and pasteurised milk. Some NTM can cause nontuberculous mycobacteriosis particularly in immunocompromised patients (e.g. HIV patients) and thus gain clinical significance.
There is no current therapeutic standard for nontuberculous mycobacteriosis. The treatment is chosen according to the respective mycobacteria species. Consequently, reliable differentiation between clinically relevant NTM species is important for the therapy regimen.
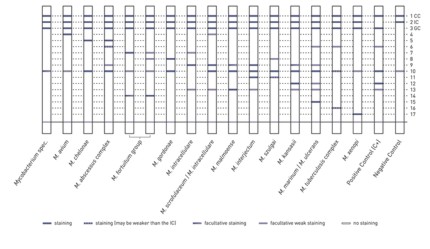
Starting from sputum specimens, GenoType CMdirect allows for the rapid and reliable differentiation of M. tuberculosis complex and NTM. Besides, the simultaneous detection of more than 20 clinically relevant NTM species is also possible.
Your benefits with GenoType CMdirect
- Maximum efficiency: For the test procedure patient specimens are used as starting material. With a single processing numerous clinically relevant mycobacteria species are detected.
- Time advantage: The results are available withinfive hours only. This means an enormous time advantage as compared to culture-based methods.
- High reliability: Several controls which are provided with the kit guarantee valid results.
- High flexibility: The test can be processed manually or automated and is suitable for low to high sample throughput.
Not all of our products are available in every country. Please contact your local sales representative for availability of this IVD product in your country.
At a glance
Molecular genetic test system for identification of clinically relevant mycobacteria
Starting material:
Decontaminated sputum specimens
DNA Isolation:
GenoLyse®
Order number
- 12 tests No. 295
DNA•STRIP technology
Downloads:
Information request:
More Information: